Published Paper: Hydrophobic gating in bundle crossing channels
Transmembrane ion channels frequently regulate ion permeation by forming bundle crossing of the pore-lining helices when deactivated. The resulting physical constriction is believed to serve as the de facto gate that imposes the major free energy barrier to ion permeation. Intriguingly, many ion channels also contain highly hydrophobic inner pores enclosed by bundle crossing, which can undergo spontaneous dewetting and give rise to a “vapor barrier” to block ion flow even in the absence of physical constriction. Using atomistic simulations, we show that hydrophobic gating and bundle-crossing mechanisms co-exist and complement one and another in the human TRPV4 channel. In particular, a single hydrophilic mutation in the lower pore can increase pore hydration and reduce the ion permeation free energy barrier by about half without affecting the bundle crossing. We believe that hydrophobic gating may play a key role in other bundle-crossing ion channels with hydrophobic inner pores. 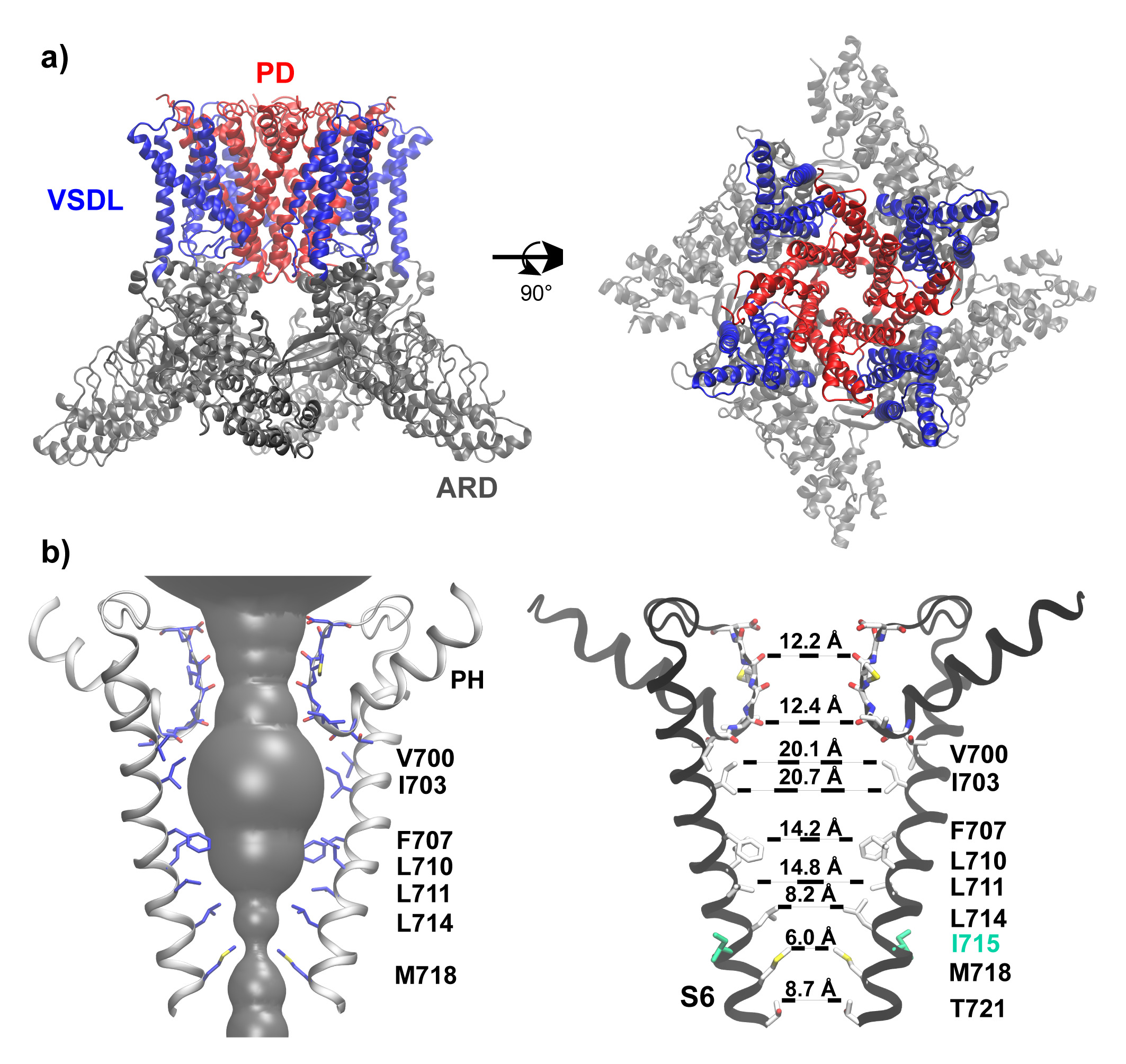
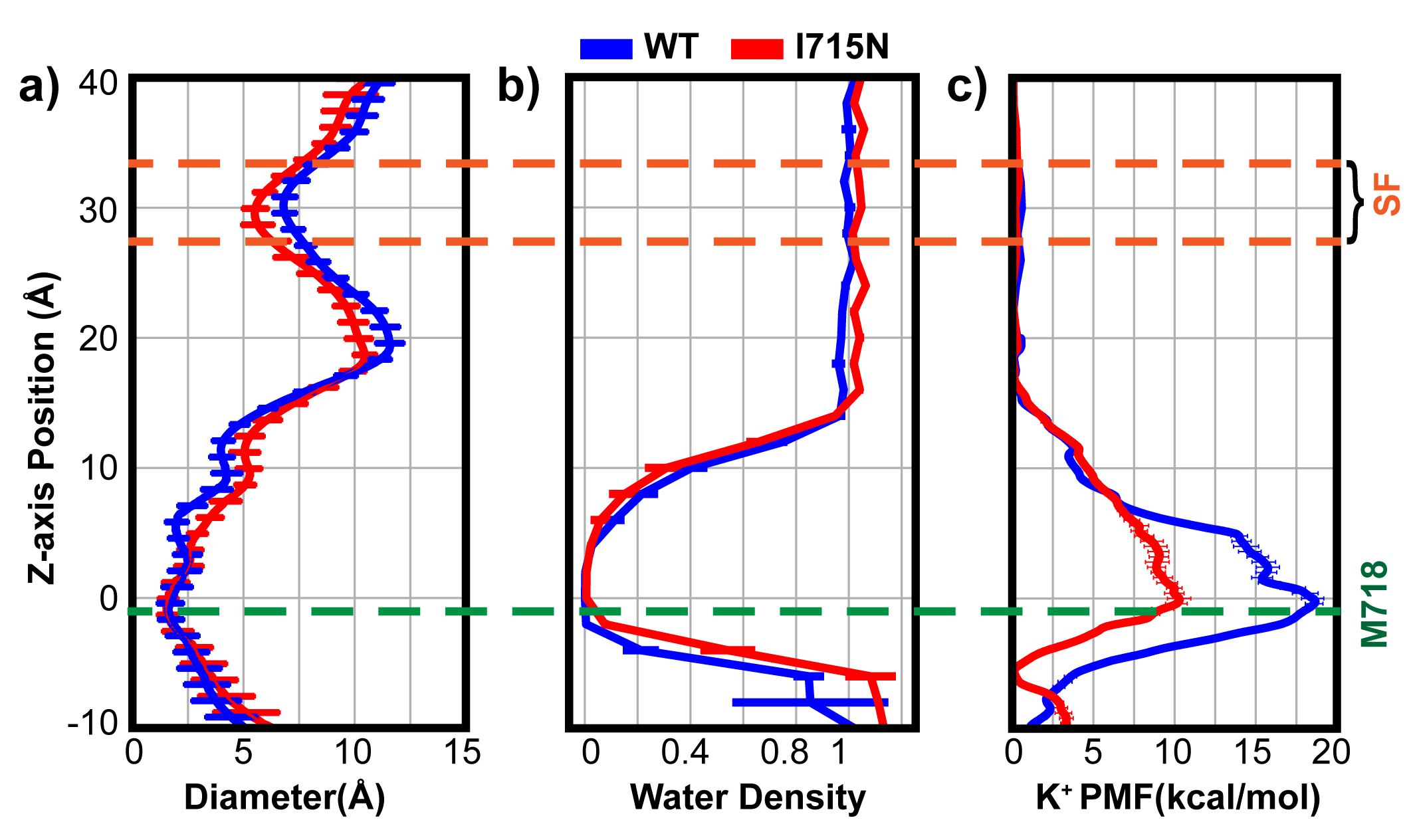
In this work, we performed atomistic molecular dynamics (MD) simulations in explicit solvent to investigate the hydration property of TRPV4 pore in the closed state, and further determined the free energy profile of K+ permeation to identify the location of the actual gate. To dissect the contributions of hydrophobic gating and bundle crossing to channel gating, we analyzed the pore hydration and K+ permeation-free energy properties of a hydrophilic and non-pore-facing mutation I715N near the bundle crossing, which was experimentally found to increase the resting channel activity of TRPV4. Our results strongly support that both bundle-crossing and hydrophobic gating indeed contribute to TRPV4 inactivation. Given the prevalence of hydrophobic inner pores in ion channels, the current study suggests that hydrophobic gating likely plays a more general role than previously thought, regardless of the presence of bundle crossing in the deactivated channel.
The above figure shows that I715N has a significantly smaller barrier compared with the WT.
Many thanks to my supervisor, Jianhan, for his support during the whole manuscript preparation and submission process.