Published Paper: Targeting TRP Channels: Recent Advances in Structure, Ligand Binding and Molecular Mechanisms
In this short review, we summarize recent progresses toward understanding the structural basis of TRP channel function, as well as potential ligand binding sites that could be targeted for therapeutics.
Transient receptor potential (TRP) channels are a large and diverse family of transmembrane ion channels that are widely expressed, have important physiological roles, and are associated with many human diseases. These proteins are actively pursued as promising drug targets, benefitting greatly from advances in structural and mechanistic studies of TRP channels. At the same time, the complex, polymodal activation and regulation of TRP channels have presented formidable challenges. A particular focus is on the current understanding of the molecular mechanisms of TRP channel activation and regulation, where many fundamental questions remain unanswered. We believe that a deeper understanding of the functional mechanisms of TRP channels will be critical and likely transformative toward developing successful therapeutic strategies targeting these exciting proteins. This endeavor will require concerted efforts from computation, structural biology, medicinal chemistry, electrophysiology, pharmacology, drug safety and clinical studies.
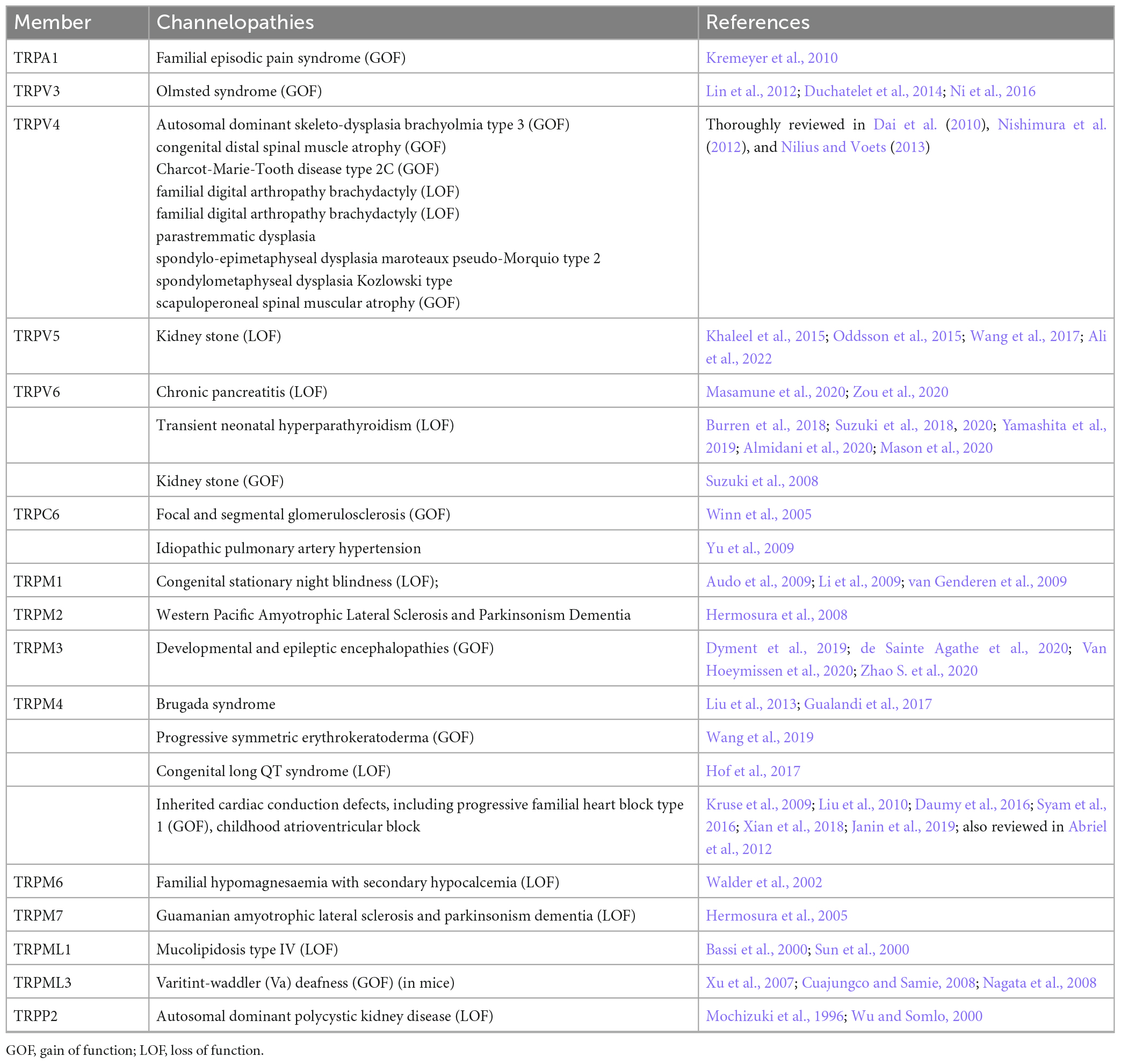
In this work, we first summarized all kinds of TRP-related genetic diseases (channelopathies) to emphasize the necessity of developping TRP-targeting drugs for therapeutics.
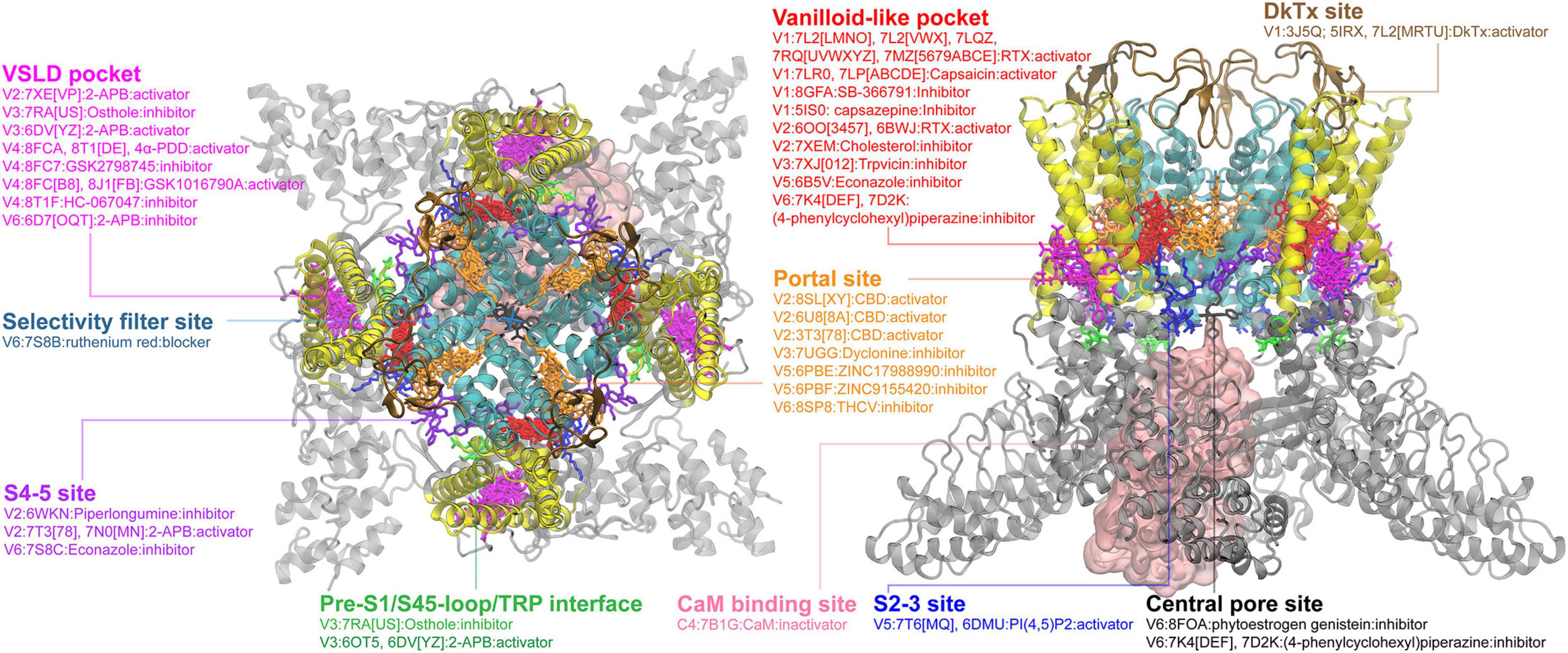
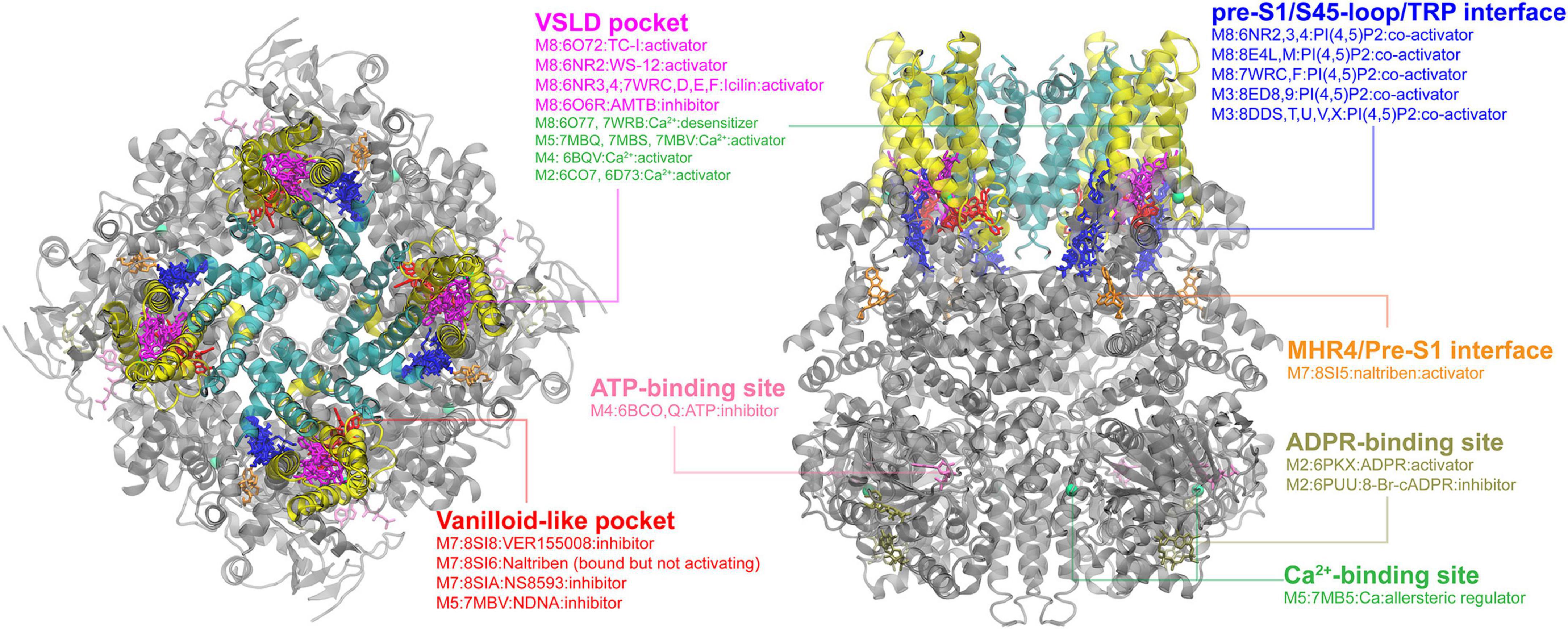
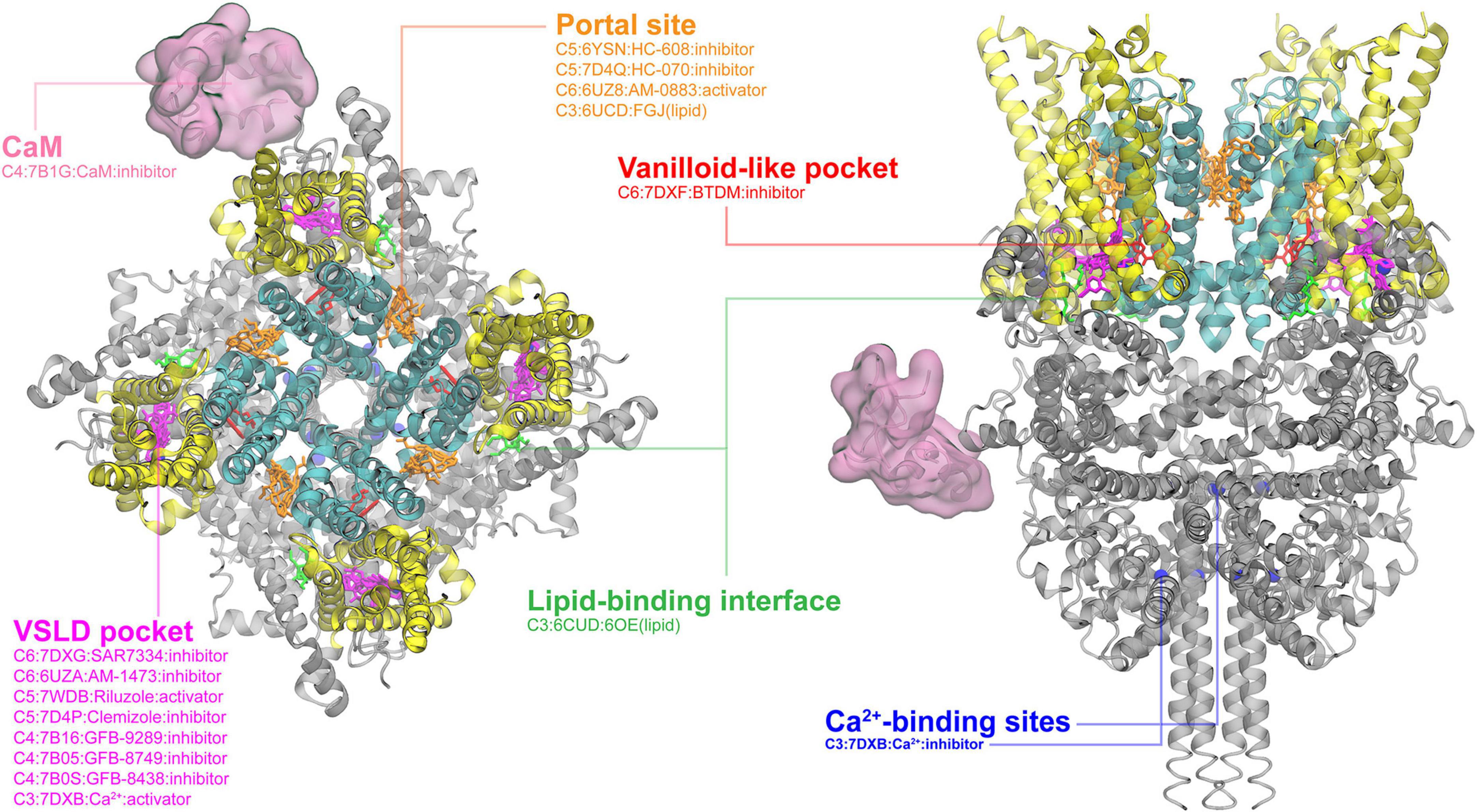
Then, we went through all six TRP subfamilies to summarize all reported ligand-binding pockets from the currectly available structural data from the PDB database. Below are the figures summarizing TRPM and TRPC binding pockets. Detailed annotations including activation or inhibition are added in the figures.
Ligand-binding pocket in TRPMs
Ligand-binding pocket in TRPCs
Many thanks to Prof. Jianhan Chen, Mahdieh Yazdani, Aron Korsunsky for their support during the whole manuscript preparation and submission process.