TMEM16F: an ion channel can scramble lipids
TMEM16F plays important roles in regulation of membrane asymmetry and associated physiological processes. Extensive structure determination efforts have failed to capture a conductive/open state of the protein, hindering our understanding of TMEM16F activation and its dual functionality as both lipid scramblase and ion channel.
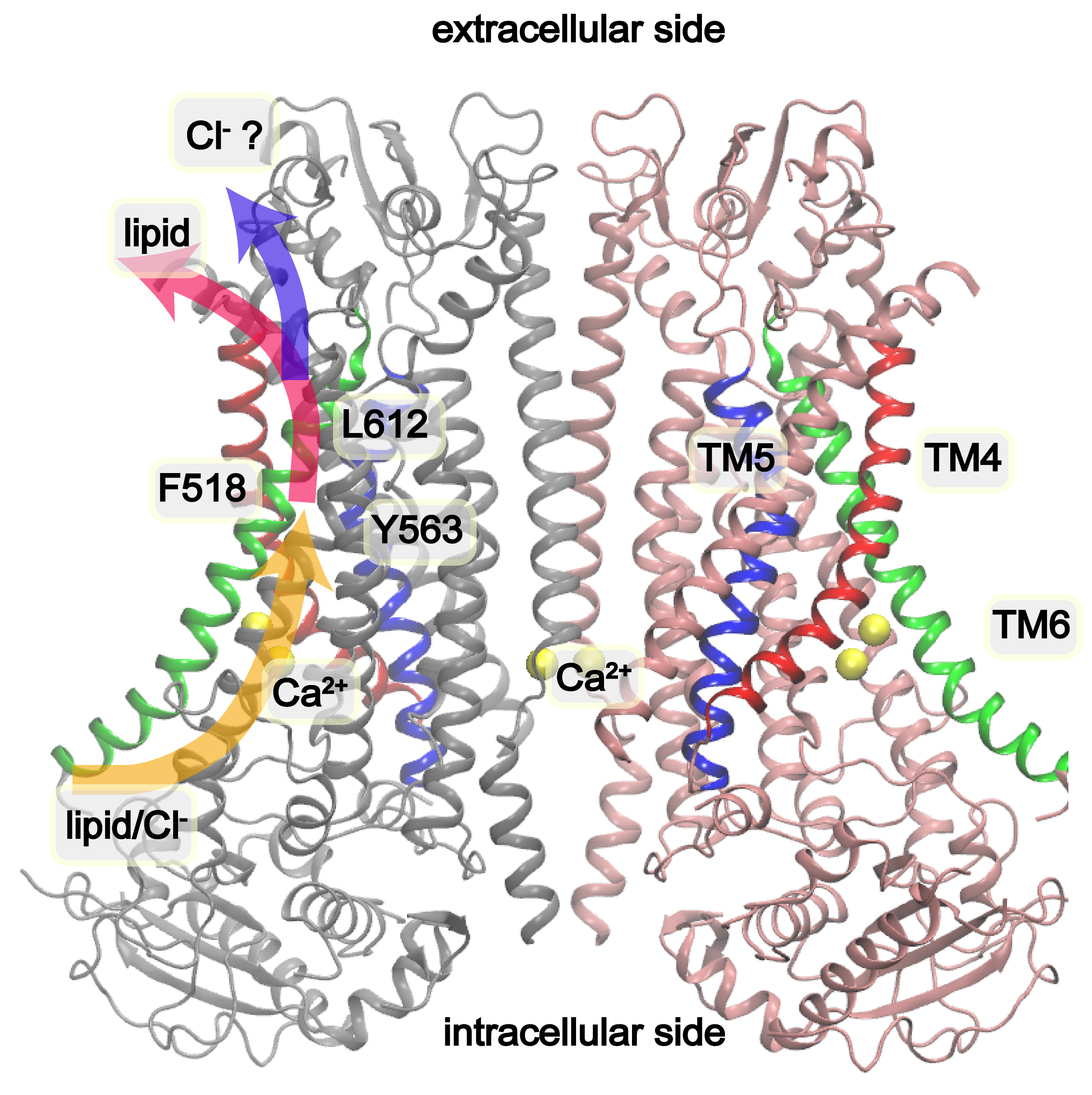
A previous study discovered that mutations of inner activation gate residues (labelled in the above picture: F518, Y563 and I612) to charged ones led to constitutively active scramblases. Leveraging this discovery, we performed multi-microsecond atomistic simulations and successfully observed spontaneous opening of the conduction pore of TMEM16F. Further analysis provides important new insights into the activation mechanism of TMEM16F and the molecular basis of its lipid scramblase and ion channel function.
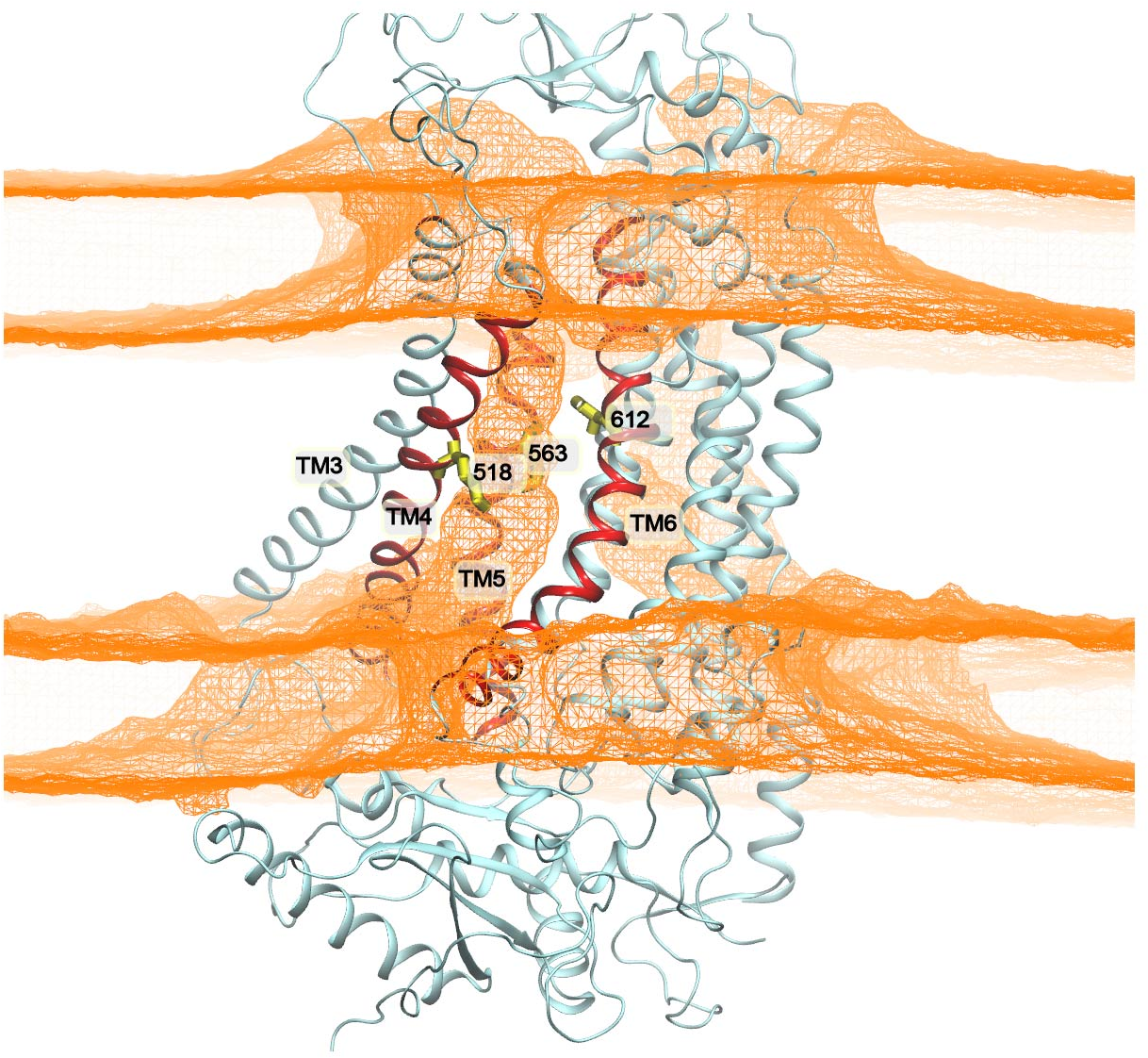
The inner gate double charged mutant (F518K-Y563K) is permeable to lipids (shown by density map colored in yellow mesh) during our simulation!
